Master and PhD projects from the lab
Here we present Master and Phd projects from the lab and post corresponding pdf files
Manni, L. Adrenoceptors and Nerve Growth Factor: Effects of Electroacupuncture and Physical Exercise in Rats with Steroid-Induced Polycystic Ovaries. Ph.D thesis, Wallenberg Laboratory for Cardiovascular Research, Cardiovascular Institute, Sahlgrenska Academy, Göteborg University, (2005). ISBN: 91-628-6484-X.
Ph.D. Thesis Luigi Manni (2005)
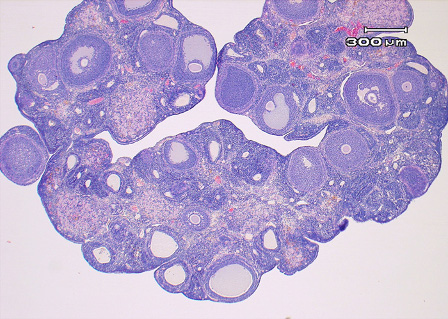
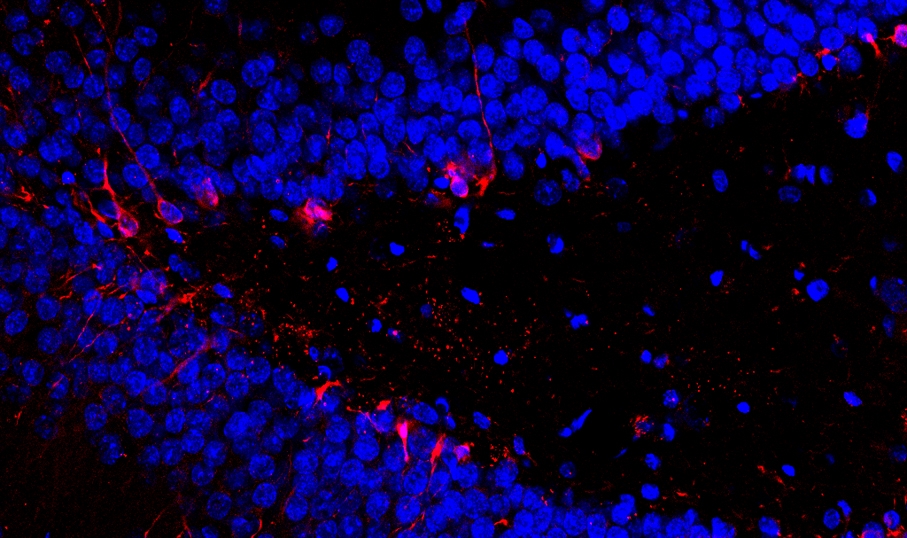
Polycystic ovary syndrome (PCOS) is a complex endocrine and metabolic disorder associated with ovulatory dysfunction, follicular cyst and hyperandrogenism. Despite extensive research the pathogenesis of PCOS is still unknown. During the last decade the hypothesis of an increased sympathetic tone to the ovaries and central sympathetic outflow in women with PCOS has gained support.
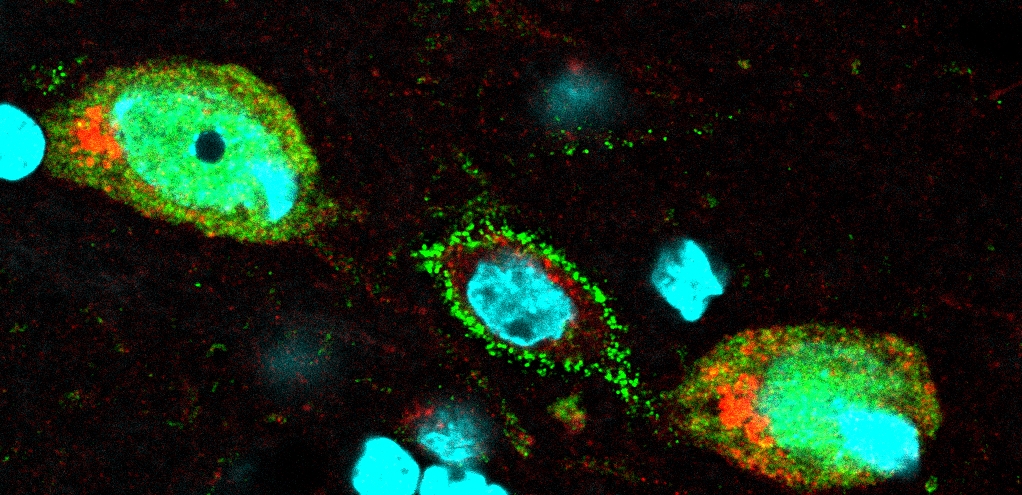
The sympathetic nerves appear to be involved in the regulation of ovarian function. Recent work on sympathetic-related mechanisms underlying the development of steroid-induced PCO points to a hyper-responsiveness of system in mediating norepinephrine (NE) signaling to the ovaries. It has also been demonstrated that the development of ovarian follicular cysts is preceded by an increased synthesis of ovarian nerve growth factor (NGF) and p75NTR mRNA in rats with steroid induced PCO. Our studies demonstrates that:
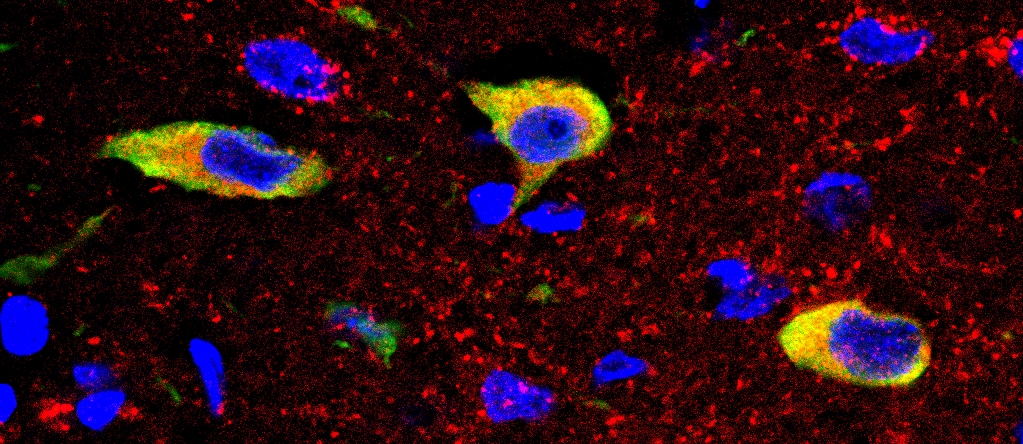
Rats with estradiol-valerate (EV)-induced PCO has not only deregulated intra-ovarian β2-adrenoceptors (AR), tyrosine hydroxylase (TH) and p75 neurotrophin receptor (p75NTR) expression, but also deregulated expression of all α1-AR subtypes during the first 30 days (early phase) after EV-injection.
Blockade of endogenous NGF action restores the EV-induced changes in ovarian morphology and expression of the sympathetic markers α1- and β2-AR, p75NTR, NGF-tyrosine kinase receptor (TrkA) and TH.
Repeated electroacupuncture (EA) treatments decreased ovarian NGF and p75NTR protein and counteracted the EV-induced changes of α-AR and β2-AR.
Ovarian morphology was almost normalized and the EV-induced increase of ovarian NGF and p75NTR as well as the deregulated ovarian ARs was counteracted by physical exercise. Taken together our results suggest that EA and/or exercise could be effective in the treatment of anovulation and possibly in the prevention of human PCOS.